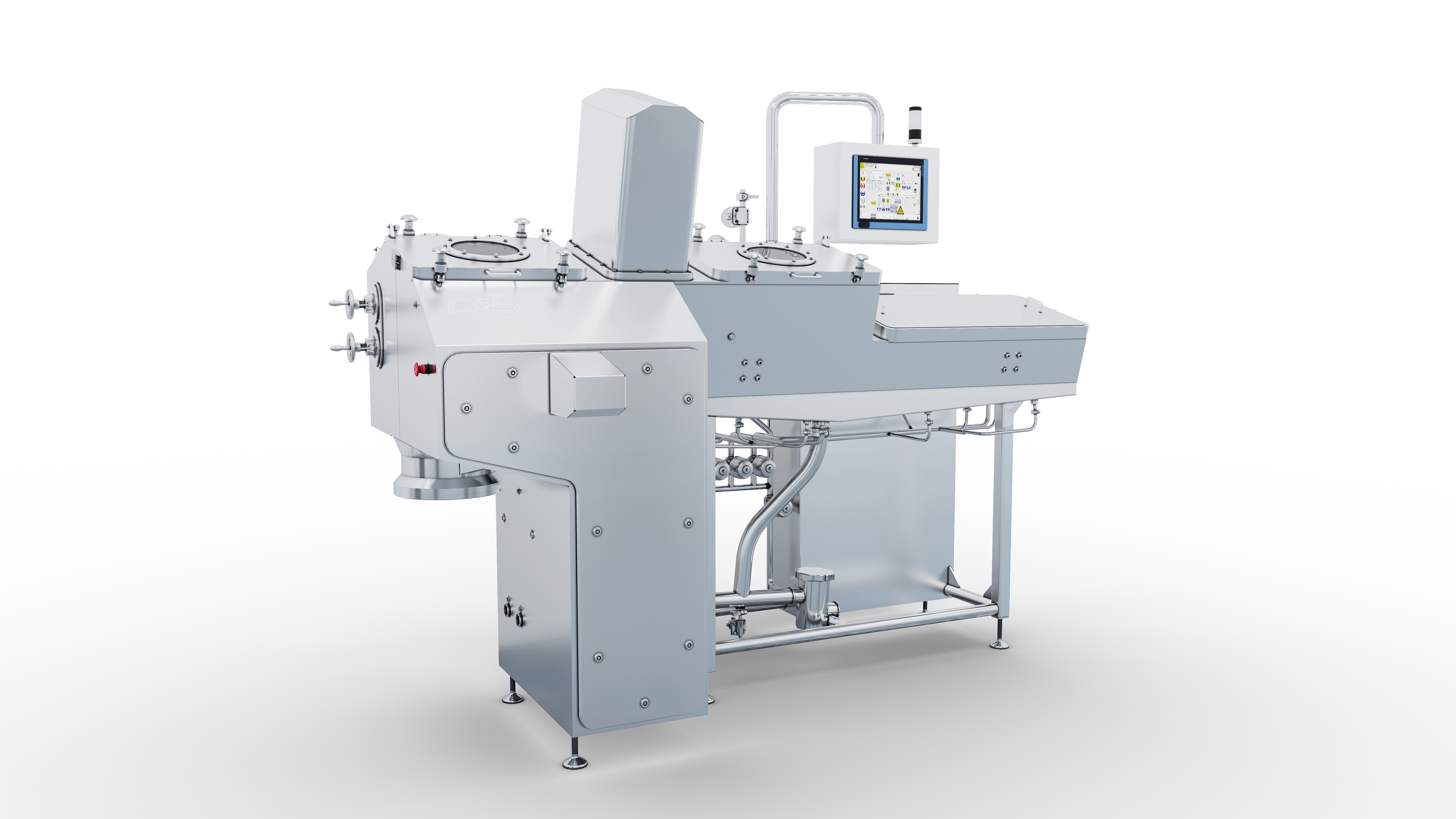
C&S serves the high-level biopharmaceutical industry, providing fermenter, bioreactor, ultrafiltration module, inline dilution and inline conditioning system (ICS) module, CIP module, blood-plasma-bag cutting and emptying machine and other professional equipments, as well as upstream and downstream process system integration, the whole plant digitization, information and automation & control solution in the application of monoclonal antibody, human blood plasma, vaccine, insulin and formulation. Our service covers process conceptual design, basic design, detailed design, vessel manufacturing, module and cabinet assembly, on-site installation, commissioning and validation, training and after-sales service.
We have been committed to providing customers with state-of-the-art technology and solutions. The Engineering team has many years of experience in process design and automatic programming and commissioning in high-level biopharmaceutical projects, is familiar with international and domestic regulation, production requirements
and operating habits in biopharmaceutical factories. C&S Strictly follows the ISPE and ASME BPE standards to guide engineering design, and brings the advanced concept and the latest global industry trends into the design to ensure that the design meets Chinese cGMP, EU and FDA regulation and continuously creates maximum value for customers.