Quality by design (QbD) is adopted by large global biopharmaceutical companies. Under the guidance of this principle, biopharmaceutical process development and production are carried out. The same concept applies to pharmaceutical equipment manufacturing and engineering execution.
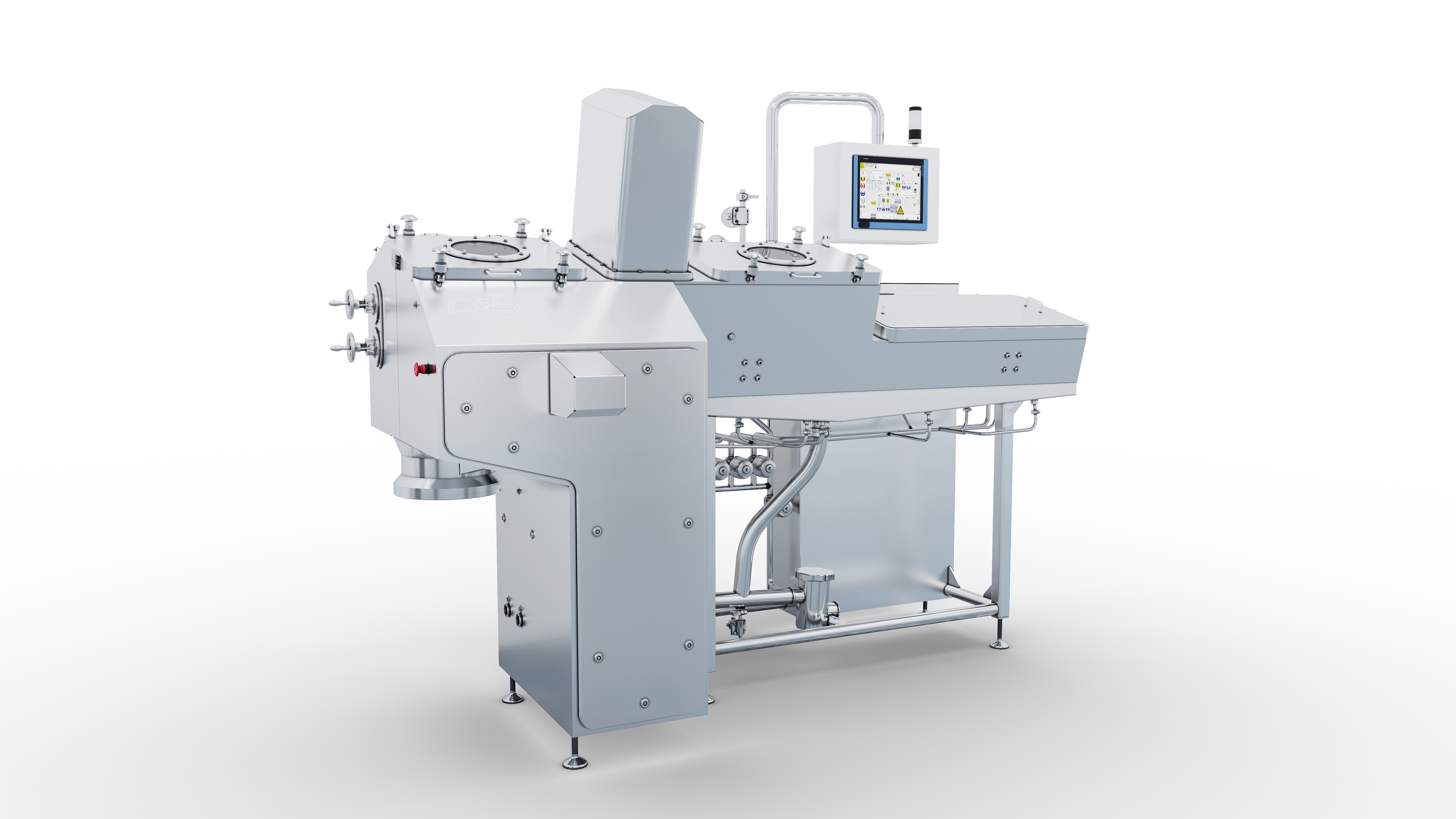
C&S’s main engineers come from a professional European engineering company with a century of engineering technology experience. They have rich experiences in executing high-level projects in biopharmaceuticals, are familiar with process requirements and operation in biopharmaceutical facilities to ensure that the customized design meets the requirements of Chinese cGMP, and also achieves the concepts of EU and FDA. Our design can cover conceptual design, basic design, and detailed design.