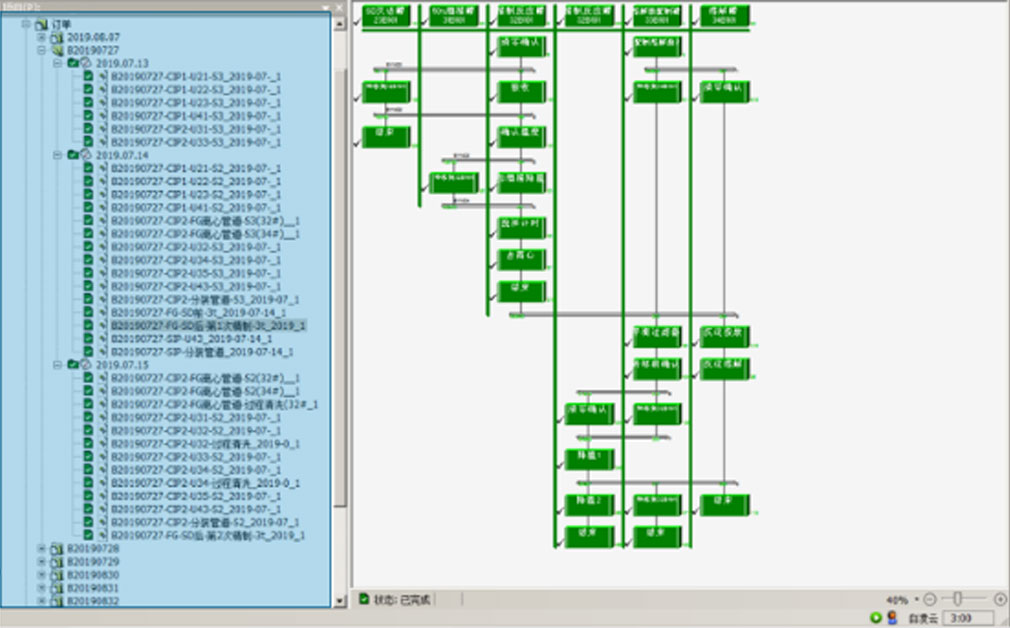
The batch control management on the whole process realizes the automation and control execution of the whole process.
The automation and control system with compliance, advanced and user-friendly interface is the core of the operation of the biopharmaceutical system. It can not only continue to optimize the the production process with multi-batch operation data record and analysis, but also ensure the stability of the product quality and the reproducibility of the production parameters, eliminate operator’s mistakes in the production process and reduce the production cost. Our automation engineers are familiar with its production process and automation and control technology, and can design PLC control system and DCS system according to the users’ unique process and operation. The batch control system based on ISA 88 principle is developed to meet the requirements of FDA 21CFR PART 11 and computer validation.
The batch control management on the whole process realizes the automation and control execution of the whole process.
The Modular design of electrical and mechanical structure ensure module delivery after full FAT.